

Meningitis (epidemic cerebrospinal meningitis) is an acute respiratory infectious disease caused by meningococcal infection of the cerebrospinal membrane. It is mainly transmitted from the air by droplets, such as coughing and sneezing. The source of infection is mainly patients and carriers.
The epidemic cerebrospinal meningitis has the characteristics of rapid onset and various changes, the severe could lead to death within 24 hours after the onset of the disease (accounting for about 10 % of the number of cases). Even after cure, some patients will have sequelae, mainly showing as brain damage, hearing loss, deafness and mental retardation.
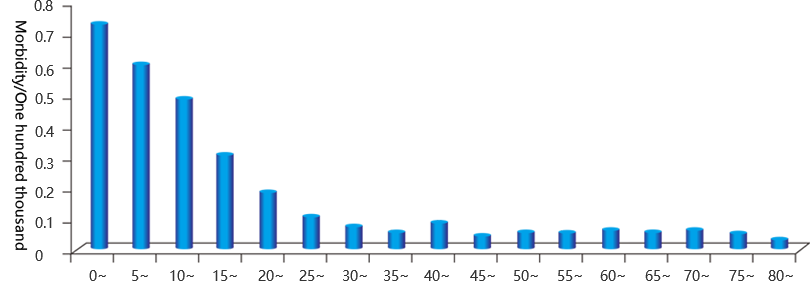
Children and adolescents are susceptible to epidemic cerebrospinal meningitis
China is one of the countries with a high incidence of epidemic cerebrospinal meningitis. In recent years, the incidence of epidemic cerebrospinal meningitis is about 0.25 / 100,000. The age group of this disease is still 0-19 years old, accounting for 77.40 % of the total cases.
References: Li Yixing, Shao Zhujun, etc. Epidemiological characteristics of epidemic cerebrospinal meningitis in China in 2005/2006 [ J ]. China Planned Immunization, 2007, 13 (3): 193 - 196.
The main pathogenic strains of epidemic cerebrospinal meningitis were group A, B, C, Y and W135. Because of the epidemic flora of epidemic cerebrospinal meningitis can change, it brings great challenges to the prevention and control work.
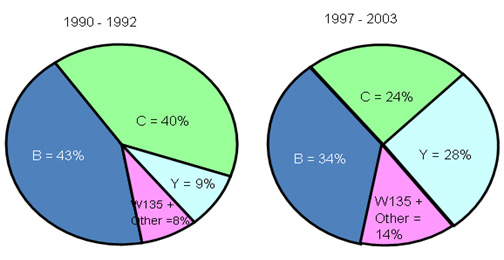
Changing Meningococcal Serogroup Distribution in the U.S.*
The proportion of meningococcal flora in the United States is changing, with Y and W135 rising. It shows that the dominant flora of epidemic cerebrospinal meningitis changes very rapidly
Before the 1950s, the epidemic cerebrospinal meningitis in the United States was mainly caused by group A, which was gradually replaced by group B after 1954. After 1967, the main bacteria strain was group C. In 1973, the disease was caused by group B, C, Y, A and W135. Today, the epidemic cerebrospinal meningitis in the United States is dominated by group Y, followed by groups B and C, with only about 1% of group A strain isolated from epidemic cerebrospinal meningitis patients.
In addition, group A bacteria strains caused the outbreak of meningitis in Mecca pilgrims in 1987, but group W135 caused the outbreak in Mecca pilgrims in 2000.
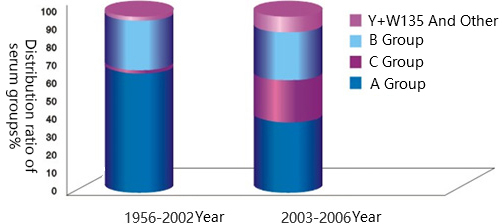
References: Shao Zhujun, Xu Li, etc. Analysis of the Change Trend of Epidemic Flora of Epidemic Cerebrospinal Meningitis in China [ J ] Planned Immunization in China, 2007,13 ( 6 ): 541-544
Before 2002, the epidemic cerebrospinal meningitis in China was mainly caused by group A. Since 2002, Guangxi has discovered the first case of epidemic cerebrospinal meningitis caused by group C which confirmed by etiology, then several local outbreaks were caused by group C, and no epidemic outbreak has occurred after vaccination. In 2008, the first case of W135 meningitis confirmed by etiology occurred in Guangdong, China. With more and more close communication between China and the world (such as the Olympic Games and World Expo) and the increasingly population movement in various parts of the country, the probability of the introduction of new epidemic groups Y and W135 into China increases, because the vaccines previously vaccinated can only prevent groups A and C, so the epidemic of groups Y and W135 may occur locally in China.
After years of painstaking research and development, the company has developed group ACYW 135 meningococcal polysaccharide vaccine, which is higher than the European Pharmacopoeia standard


The incidence of local adverse events is less than 10 / 100,000

Good immunogenicity and high seroconversion rate


It not only provides protection for Y and W135 groups, but also strengthens the immunity of A and C groups.
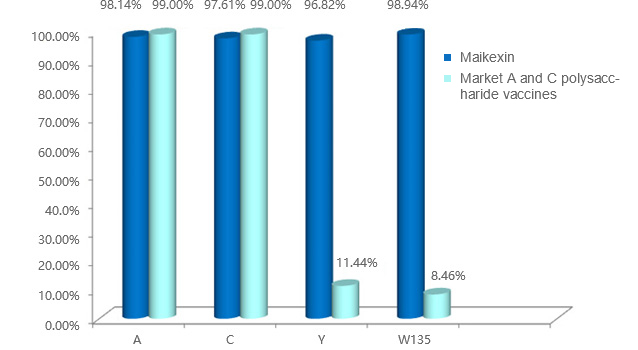
Li Rongcheng, Report on Phase III Clinical Trial of (A + C + Y + W135) ACYW 135 meningococcal polysaccharide vaccine. Guangxi CDC