

Rabies is a viral zoonosis with great harm. It has the highest fatality rate (100 %) in history. Rabies has a long history and has long been described and recorded in ancient Egypt and the Spring and Autumn Period in China.
Rabies is popular in most countries among the world. The World Health Organization estimates that 55,000 people worldwide died of rabies annually, 99 % of which occur in developing countries, while Asia is the region with the most serious rabies epidemic in the world, accounting for about 56 % of the total number of cases worldwide. Rabies poses a great potential threat to more than 2 billion people living in epidemic areas in Asia.
China's rabies vaccine embraces the "human" era
1.Production with healthy human cell, innovated processing, highly purified
2.Fast acting, and the average antibody level in 14th days is nearly 40 times ofseroconversion level
3.The duration of immunization lasts for 8 years
4.Less than 1% of ADRs,good safety

Comparison of Human-derived and Animal-derived Matrix Vaccines

“General Principles for Evaluation of Cell Matrix for Vaccine Production” by Drug Review Center


Imported raw materials, innovated processing, highly purified


Bioreactor microcarrier culture

Purification: Molecular sieve column chromatography

Average antibody level ( GMT )in day 42 is nearly 75 times of seroconversion level
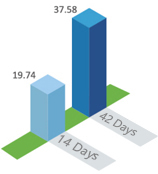 antibody level GMT(IU/ml)
antibody level GMT(IU/ml)The antibody seroconversion rate can reach to 100% in day 14
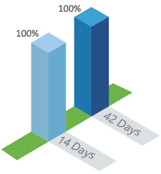 antibody seroconversion rate
(100%)
antibody seroconversion rate
(100%)In 2008, a phase III clinical trial of Kangh HDCV was conducted by Jiangsu Center for Disease Control and Prevention,including 1200 subjects aged 10 to 60 years with high-risk rabies infection, randomly divided into groups, of which 60 subjects were vaccinated with HDCV according to the post-exposure procedure.
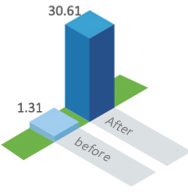
8 years after the vaccination, the average antibody level ( GMT ) was still as high as 1.31 lU / ml
 antibody levelGMT(IU/ml)
antibody levelGMT(IU/ml)
In 2016, the Lianshui Center for Disease Control and Prevention in Jiangsu Province randomly selected 60 volunteers who had participated in the Phase I clinical trial of Kangh HDCV and not immunized rabies vaccine during this period to conduct the 8 years’ immune persistence observation. The test results showed that after 8 years of vaccination with Kangh HDCV, 46.67% of the volunteers were still at the positive level of antibody (>0.5IU/ml), and the GMT was 1.31 IU/ml. After one dose of booster vaccination, the GMT rapidly increased to 30.61 IU /ml.


The common adverse reactions include:
Pain and induration at injection site, rash, headache and nausea
In 2016, the canine injury clinic of Shijiazhuang Center for Disease Control and Prevention took 1040 patients who vaccinated with Kangh HDCV as the research objects to conduct a safety statistical study. The results showed that the total number of adverse reactions was 9 cases, no adverse reactions of grade 1 or above occurred, and the adverse reaction rate was as low as 0.87 %.
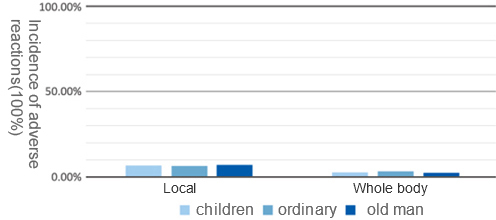
In 2015, the canine injury clinic of Urumqi Center for Disease Control and Prevention took Kangh HDCV recipients as the research object to observe and analyze the safety after vaccination. A total of 372 cases were included, divided into children group, normal group and elderly group. The results showed that the total adverse reaction rate was low, and there was no significant difference in the incidence of local and systemic adverse reactions among the groups (P > 0.05). No adverse reactions above grade 1 occurred after inoculation, and all adverse reactions could relieveby self within 72 hours.